Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine
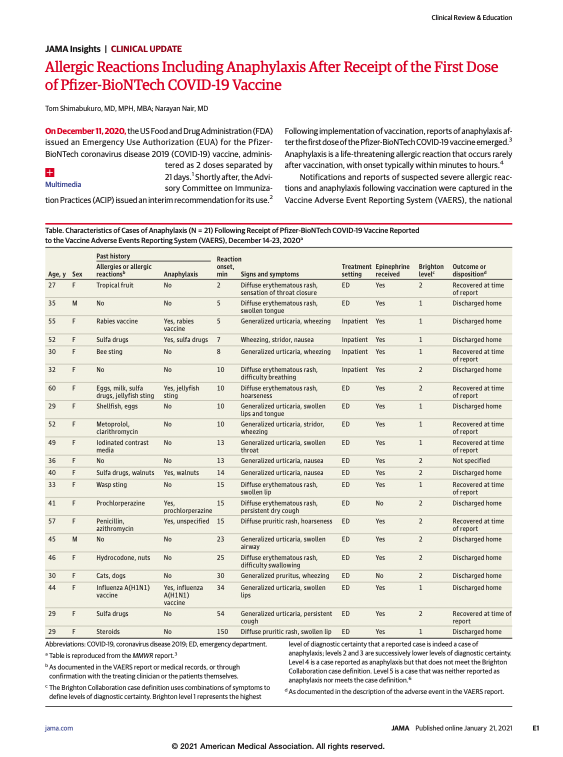
On December 11, 2020, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the Pfizer-BioNTech coronavirus disease 2019 (COVID-19) vaccine, administered as 2 doses separated by 21 days. Shortly after, the Advisory Committee on Immunization Practices (ACIP) issued an interim recommendation for its use. Following implementation of vaccination, reports of […]